November 23, 2022
Highlights and Key Updates
- Nationally, 77.7% of pediatric beds are occupied, up 1.7% from 76% on 11/16. As of 11/20, 30 states show increases in in-patient pediatric bed utilization, with highest rates in: Arizona: 98.87% (-0.13%), Rhode Island: 96.17% (+2.17%), Utah: 95.90% (+1.90%), District of Colombia: 95.75% (+3.75%), Kentucky: 93.41%, Maine: 90.43%, Minnesota: 90.39% (-0.61%), Oregon: 90.12%, Idaho: 89.09%, and Texas: 89.03% (-0.97%).
- Amoxicillin remains in short supply. At least one US based company has reached out to the White House to indicate greater capacity for manufacturing and storage.
- Massachusetts and Colorado have joined other states or jurisdictions in expanding or amending COVID-19 policies and emergency measures to address the surge.
- There are reports of rising rates of pertussis (a vaccine-preventable respiratory illness) in parts of California, New York, and Texas.
- COVID-19 vaccination rates for children vary widely across states, ranging from 2% to 37% having received their first dose according to analysis of CDC data by AAP. Policies and messaging aimed at increasing COVID-19 vaccination rates for eligible infants could be a powerful way to reduce their risk of severe illness and hospitalization, which could mitigate additional surge.
- As of 11/23, there is no response to AAP and CHA’s November 15 letter calling for government officials to declare a public health emergency for RSV.
- Several manufacturers report limited or no supply of certain oseltamivir formulations (an influenza antiviral).
Healthcare Ready is
ENGAGED for this event.
We are monitoring potential concerns for supply chain disruptions and impacts on healthcare services on our Pediatric Surge in Respiratory Illness response page, as well as listing resources and previous situation reports.
Assessment of Healthcare and Logistics Impacts
Background
The confluence of RSV, influenza, and COVID-19 is creating a surge in severe pediatric respiratory illnesses and hospitalizations that threaten healthcare delivery systems. Influenza and RSV activity are higher than usual for the time of year, perhaps due to pandemic related preventative measures being relaxed. It is not yet clear how the surge in respiratory illnesses will impact the capacity of facilities, such as community health centers, free and charitable clinics, urgent care, or pharmacies. These facility types will be critical for case identification and first-line treatment.
Healthcare Ready is working to understand these impacts to best support communities with the greatest needs.
Pediatric Hospitalizations
- Nationally, 77.7% of pediatric beds are occupied (up 1.7% from 11/16).
- As of 11/20, states with the highest rates of in-patient pediatric bed utilization are: Arizona: 98.87% (-0.13%), Rhode Island: 96.17% (+2.17%), Utah: 95.90% (+1.90%), District of Colombia: 95.75% (+3.75%), Kentucky: 93.41%, Maine: 90.43%, Minnesota: 90.39% (-0.61%), Oregon: 90.12%, Idaho: 89.09%, and Texas: 89.03% (-0.97%). See below for a map of pediatric hospital bed utilization.
- 30 states and territories show increased rates of pediatric bed utilization compared to 11/16. States with the greatest increases compared to 11/16, include: North Carolina: 78.53% (+21.53%) and Vermont 76.74% (+17.74%).
- 24 states and territories experienced decreases in pediatric bed utilization. Of note, Delaware’s pediatric bed utilization rate fell 8.56%, from 88% on 11/16 to 79.44% on 11/20.
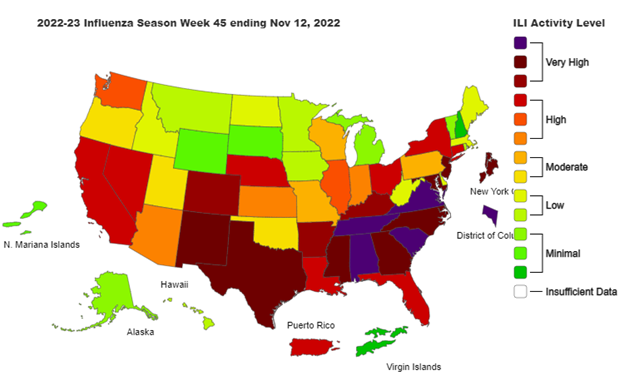
Source: CDC Weekly US Influenza Surveillance Report. Accessed 11/18/2022.
COVID-19
- The overall rate of new US COVID-19 hospitalizations for all age groups is lower than at the beginning of the year.
- National-level data shows a very slight increase in new hospital admissions of pediatric patients with confirmed COVID-19 from the prior week. New admissions of elderly patients also increased slightly. As of 11/20:
- People ages 0-17: +0.4% new admissions per 100,000
- People ages 70+: +1.4% new admissions per 100,000
Epidemiology Updates for Respiratory Illnesses of Concern
Hospitalizations and case rates for COVID-19 and influenza are tracked separately from RSV cases. This makes it difficult to discern the number of hospitalizations caused by each virus in each state, which may make it more difficult for jurisdictions to predict surges for each condition.
CDC tracks cases in three ways, by: State, HHS Region, and census region.
Data for RSV cases and hospitalizations for the week ending 11/18 will be updated by CDC on 11/25 or 11/28. As a result, RSV case and hospitalization data reporting in this document remains the same as last week. From CDC’s RSV-NET* for week ending 11/11:
- The youngest populations continue to experience the highest hospitalization rates.
- The hospitalization rate for children aged 0 to <6 months was 171 per 100,000 (- 14.2 compared to the previous week, but still almost triple the rate of last year).
- Hospitalization rates for all ages remain high for:
- Hispanic individuals (3.5 hospitalizations per 100,000)
- American Indian/Alaska Native (3 hospitalizations per 100,000)
- Compared to White individuals (1.9 hospitalizations per 100,000)
Potential Threats for Pediatric Medical Surge
Several challenges are unique to managing pediatric medical surges, particularly for the healthcare workforce and supply chain. For one, pediatric hospitals require more intensive nursing resources to treat and monitor patients, especially in intensive care and neonatal intensive care units.
Additionally, pediatric supply chains can also be more vulnerable to supply chain disruptions, as some critical products have only one supplier or manufacturer capable of producing the necessary pediatric-specific equipment and supplies.
Product Availability
- Definitions for product shortage varies by organization. Healthcare Ready sources data from multiple organizations that maintain drug shortage lists, including:
- American Society of Health-System Pharmacists (ASHP), which defines a drug shortage as “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”
- US Food and Drug Administration (FDA), which defines a drug shortage as “a situation where the total supply of all versions of the approved product available at the user level will not meet the current demand, and a registered alternative manufacturer will not meet the current and/or projected demands for the potentially medically necessary use(s) at the user level.”
- Amoxicillin remains in short supply.
- On November 7, USAntibiotics, the sole-licensed American manufacturer of penicillin-based Amoxicillin and Amoxil Clavulanate (Augmentin), noted that they have reached out to the Biden Administration to inform them that every dose of Amoxicillin that will be needed in the US over the next five years can be manufactured and stored at their facility in Tennessee.
- Physicians should be aware of alternatives for amoxicillin when prescribing it to their patients. Some manufacturers have placed limits on the amount of pharmacies can order to respond to this shortage.
- ASHP’s current drug shortages list includes the following drugs that could negatively impact treatment of RSV and other respiratory illnesses:
- As of 11/21, Sodium chloride solution of various formulations for injection from Fresenius Kabi and Pfizer. The reasons for the shortage are manufacturing delays. Resupply dates are anticipated at the end of November for Pfizer and early December for Fresenius Kabi.
- As of 11/21, Rocuronium injection, used during tracheal intubation, is in shortage from several manufacturers due to increased demand and manufacturing delays. Estimated resupply dates vary based on manufacturer.
- As of 11/21, certain formulations of Oseltamivir, commonly known as Tamiflu, have been reported in short supply by several manufacturers. The FDA has yet to report a national shortage of this drug as they believe other manufacturers can meet demand. As this antiviral is used to treat influenza, physicians may need to prescribe other medications if their patients cannot find Tamiflu in pharmacies.
- FDA’s drug shortage database list the following updates regarding drugs that may be related to treating respiratory illness:
- As of 11/21, Amoxicillin oral powder for suspension is available for current customers from Hikma pharmaceuticals. However, as of 11/21, most of the amoxicillin oral powder product for suspension from Sandoz is unavailable.
- As of 11/21, albuterol sulfate, a bronchodilator for oral inhalation, manufactured by Akorn Pharmaceuticals, remains unavailable and is estimated to be back in stock by Q2 2023. A 5 mL version from Nephron Pharmaceuticals is available.
According to some pediatric clinics, RSV, influenza, and COVID-19 testing kits have been on backorder.
Treatments for RSV
A monoclonal antibody therapy called palivizumab is available as a precautionary measure to prevent severe RSV illness in certain infants and children at high risk for severe disease during the normal respiratory season. It cannot cure or treat children who are already suffering from severe cases of RSV; it is a preventative treatment.
- On 11/17, AAP updated its guidance: Given the known efficacy of palivizumab along with the unpredictable surge capability of RSV, AAP recommends programmatic consideration of providing more than five consecutive doses of palivizumab depending on the duration of the current RSV surge in a particular region of the country.
- Palivizumab is sold under the brand name Synagis, and is marketed by Sobi in the United States. Sobi purchased US rights to Synagis from AstraZeneca in 2018. Before COVID-19, physicians prescribed Palivizumab more frequently as a preventative measure, yet, this treatment strategy slowed during the pandemic.
- AAP says that it recommends Palivizumab in eligible infants in regions that are experiencing high rates of RSV and that it will release updated guidance as they monitor the seasonal trends.
While there is not yet a vaccine for RSV, on 11/1,
Pfizer announced that they would seek FDA approval for their RSV vaccine by the end of 2022.
Workforce ShortagesOngoing
workforce shortages may threaten the ability for facilities to establish a predictable quality of care for patients. Because pediatrics is a specialty practice, there may be
additional strain on the workforce with pediatric care experience. Reports indicate that
pediatricians are requesting increased federal support as they deal with RSV, COVID-19, and influenza treatment in unison. Physicians state that they can only successfully handle this "tripledemic" with the assistance of a federal emergency declaration and dissemination of support.
Hospitals and other healthcare facilities may need
to increase surveillance for respiratory illnesses among staff to reduce spread and the potential for staff being out sick.
Practitioner mental health should also be considered and protected.
Additional training and support for practitioners that are not used to caring for acute pediatrics cases for prolonged periods should be provided whenever possible.